Feds: QOL Medical Paid “Kickbacks” in the Form of Free Breath Testing Kits

Frederick Cooper and QOL Medical have agreed to pay $47 million to settle allegations of kickbacks. The kickbacks came in the form of “free” sucrase tests that prosecutors argued inappropriately affected medical diagnosis and treatment.
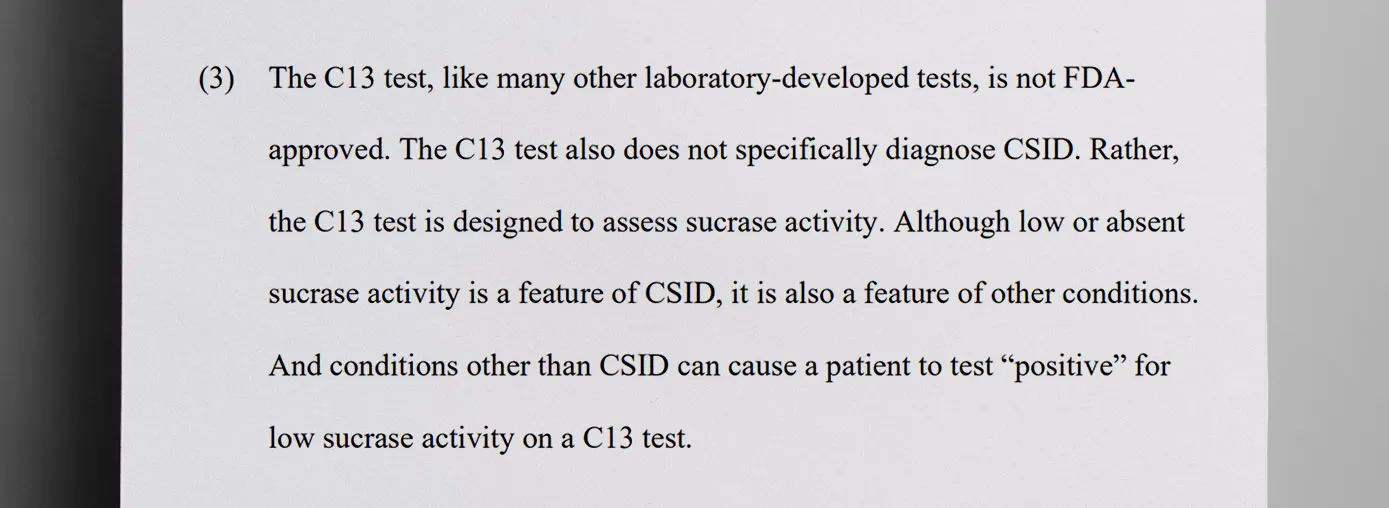
Sucraid is an FDA-approved therapy for Congenital Sucrase-Isomaltase Deficiency (CSID), a rare genetic condition that causes difficulty digesting sucrose and chronic gastrointestinal symptoms such as diarrhea, abdominal pain, bloating and gas. The C13 test is used to “assess sucrase activity” but is not approved by the FDA, according to a plea agreement obtained by The Daily Muck.
Free Breath Tests Did Not Accurately Predict C-13
It all started in 2018, when QOL, with Cooper’s approval, distributed free Carbon-13 breath test kits to healthcare providers and asked providers to give the kits to patients with common gastrointestinal symptoms. QOL claimed that the test could “rule in or rule out” CSID when, in fact, the test does not specifically diagnose CSID. Conditions other than CSID can cause a patient to test “positive” for low sucrase activity on a Carbon-13 breath test. Approximately 30% of the Carbon-13 breath tests from QOL were positive for low sucrase activity.
QOL paid the laboratory to analyze the breath tests, report the results to health care providers and send the results back to QOL. Although these results didn’t contain patient names, they did provide the name of the health care provider who ordered the test, along with the patient’s age, gender, symptoms and test result.
Between 2018 and 2022, QOL disseminated this information to its sales force with instructions to make sales calls for Sucraid to healthcare providers whose patients had positive Carbon-13 breath test results. QOL tracked whether sales representatives converted “positive” Carbon-13 breath tests into Sucraid prescriptions.
As QOL’s CEO, Mr. Cooper was aware of and approved the implementation and continuation of this marketing program, according to prosecutors.
Feds Crack Down Against Medical Kickbacks
This prosecution and others have resulted from authorities cracking down against False Claims Act and Anti-Kickback Statute violations.
In October, The Daily Muck reported a similar case in which a CVS-owned subsidiary agreed to pay $60 million in a Medicare Advantage kickback scheme.
In August, a Spokane medical supply company agreed to pay $225,000 to settle Medicare kickback allegations.
Comments by Officials
Kickbacks usually refer to cash payments in exchange for referrals or similar favorable actions. This case was unique because the “kickbacks” took the form of free medical tests.
Joshua S. Levy, Acting United States Attorney, commented in a statement that “not all kickbacks come in the form of cash going into a doctor’s or a patient’s pocket.”
“QOL provided free goods to doctors and patients in order to induce prescriptions for the very expensive drug QOL manufactured,” Levy said. “Here, the defendants relied on free breath tests and misleading sales tactics to drive patients to their product.”
Levy claimed this kind of behavior by drug companies drains money from federal health programs and inappropriately influences treatment decisions.
From the law enforcement side, the FBI investigated Cooper and QOL’s actions as a kind of fraud. “It is extremely important that we protect our government-funded health care programs against fraud of any kind,” said Jodi Cohen, Special Agent in Charge of the FBI Boston Division.
“Today’s settlement with QOL Medical and its CEO is the result of years of hard work by the FBI and our partners to make sure this company did not get away with offering improper incentives to boost sales of its drug Sucraid,” Cohen said.
“Let this case be a warning to others that we will aggressively pursue all those, motivated by greed, who try to unlawfully enrich themselves at taxpayers’ expense.”
Discover More Muck
First AI-Powered Lawsuit Exposes California’s Eco-Fraud Empire
Feature John Lynn | Apr 10, 2025

Former Child Soldier General Lied to Get Green Card
Report Strahinja Nikolić | Feb 27, 2025

Weekly Muck
Join the mission and subscribe to our newsletter. In exchange, we promise to fight for justice.
Weekly
Muck
Join the mission and subscribe to our newsletter. In exchange, we promise to fight for justice.





